Shapes Of Molecules A Level Chemistry
What is the shape and bond angle of a molecule with 5 bonding pairs. How do electrons arrange themselves.

Shapes Of Molecules David Read Key Aims Revise A Level Vsepr Theory Prepare For Phil Gale S Lectures On Symmetry Isomers Nmr Etc Ppt Download
Explanation based on VSEPR Valence shell electron-pair repulsion theory.

. This can assist in identifying a molecule as polar. Lone Pairs Some molecules have lone pairs not in a covalent bond. The two precursor molecules at left bind.
4 bonding pairs 2 lone pairs. The shape of a molecule or ion is governed by the arrangement of the electron pairs around the central atom. Bonding and Shapes of Molecules Paper 1.
Molecule of the Month. Remember to consider how many lone pairs of electrons and. Linear Trigonal Planar Bent Tetrahedral Trigonal Pyramidal Trigonal Bipyramidal Seesaw T-Shape Linear Octahedral.
OCR AS-Level Chemistry - Bonding and Shapes of Molecules. Polar molecules are always asymmetric. Shapes of Molecules and Ions A-level Chemistry OCR AQA Edexcel.
All you need to do is to work out how many electron pairs there are at the. The shape of a molecule is determined by the number of electron pairs in its outside shell and whether these electron pairs are bonding or non-bonding. You can predict the shape of a particular molecule by considering the number of electron pairs present around the central atom.
The Chemistry Tutor. What is the shape and bond angle of a molecule with 6 bonding pairs. Linear trigonal planar tetrahedral trigonal bipyramid octahedral.
60933 views Aug 19 2019 Shapes of Molecules and Ions in a Snap. Explanation of VSEPR Theory and worked examples for working out the shapes of. Bonding and Shapes of.
Video on Shapes of Molecules for A-level chemistry. Bonding Shapes PolarityMultiple Choice Question Walkthrough 2Question DownloadShapes of Molecules ExplainedhttpsyoutubeSkUmNLGWS5oIntermolecular. Why are bond angles between bonding pairs often reduced.
As far away as possible from each other to minimise repulsion. If anyone can say See-saw s. The Valence Shell Electron.
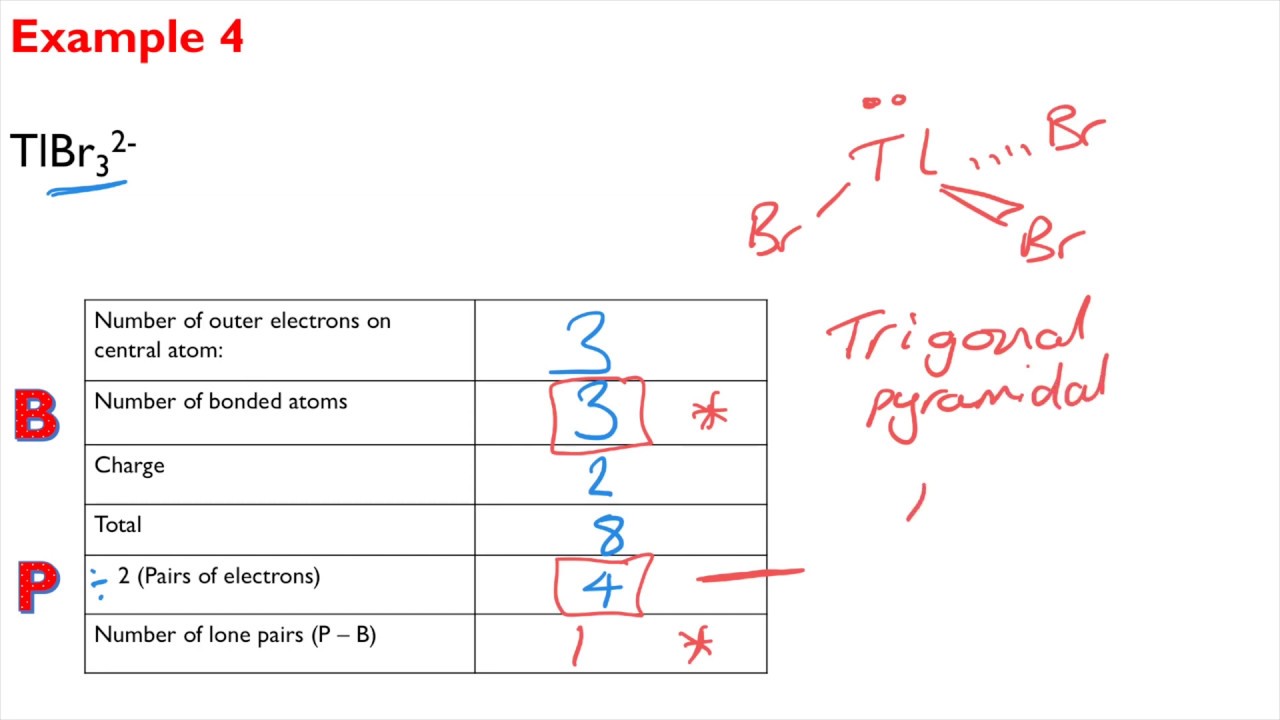
Trigonal Bipyramid- 5 Bonds. Shapes of Molecules A level Chemistry Exam Question Walkthrough. 5 bonding pairs 1 lone pair.
237904 views Mar 2 2014 This video looks at how to name and draw molecules as mentioned as part of the Bonding topic in the Physical side of the Chemistry A-Level. Has bond angle of 90 degrees between atoms on the same level and 120 degrees furthest away Shapes. These must never be overlooked during the drawing of a dot and cross diagram.
This video covers all the different shapes of molecules. Polar molecules are the result of unbalanced bond polarity and therefore all polar molecules are. The valence shell electron pair repulsion theory VSEPR predicts the shape and bond angles of molecules Electrons are negatively charged and will repel other electrons.
A modular approach to chemistry simplifies the construction of complex protein-targeting molecules.

Shapes Of Simple Molecules Chemistry
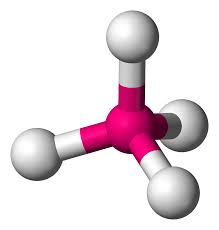
Shapes Of Molecules A Level Chemistry

3 2 Molecular Shape Atomic Combinations Siyavula

Molecular Geometry Definition Examples Video Lesson Transcript Study Com

Aqa A Level Chemistry Shapes Of Molecules Youtube

6 1 Calculation Sheet Shapes Of Molecules Pdf Chemical Bond Molecules

Shapes Of Molecules And Ions Teaching Resources
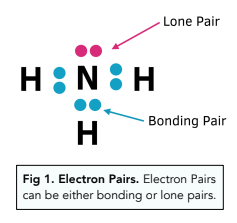
Bonding Molecular Shapes A Level Chemistry Study Mind

Aqa Jun 2011 Paper 1 Q3 With Explained Solutions
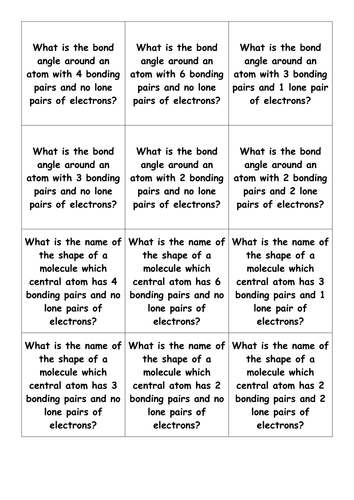
Flash Cards Shapes Of Molecules Ocr Chemistry A Level Teaching Resources

Vsepr Theory Definition Overview Expii

New Ocr Gce Chemistry A Level 6 1 Shapes Of Molecules And Ions Teaching Resources

Molecule Definition Examples Structures Facts Britannica
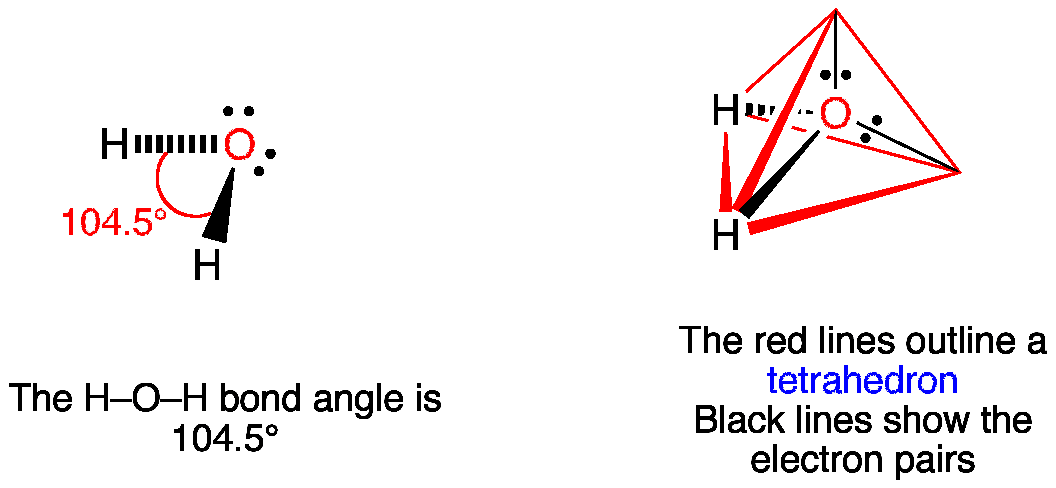
Vsepr H2o Water
Shapes Of Molecules A Level Chemistry Youtube

Chemsheets As 1017 Shapes Of Molecules Pdf Shapes Of Molecules Molecules And Ions Possess Two Types Of Electron Pairs Bonding Pairs The Two Shared Course Hero

Molecular Shapes